INTRODUCTION
While quadruplet regimens including PIs, IMiDs, and anti-CD38 mAb are now standard in the US for multiple myeloma (MM), the alkylating agent bendamustine has proven safe and effective in the first-line setting. 1 IMIDs can thus be preserved until relapse or replaced after adverse events. Carfilzomib has demonstrated efficacy in newly diagnosed MM. 2 We previously published the results of a phase I/II study of carfilzomib, bendamustine, and dexamethasone (KBD) in newly diagnosed MM during the pre-daratumumab era. 3 We report long-term survival outcomes.
METHODS
Patients enrolled in NCT02002598 with newly diagnosed MM and adequate renal function received IV carfilzomib, IV bendamustine, and IV/PO dexamethasone at prespecified doses for 8 cycles, each 28 days. 3 Stem cell collection for autologous stem cell transplant (ASCT) was performed after cycle 4. ASCT was performed after cycle 8 for transplant eligible patients. Maintenance was carfilzomib 36 mg/m 2 every 2 weeks or investigator's choice. Trial cessation occurred 12/2018. Here we provide an updated analysis of the outcome of patients with a median follow up of 77.5 months.
RESULTS
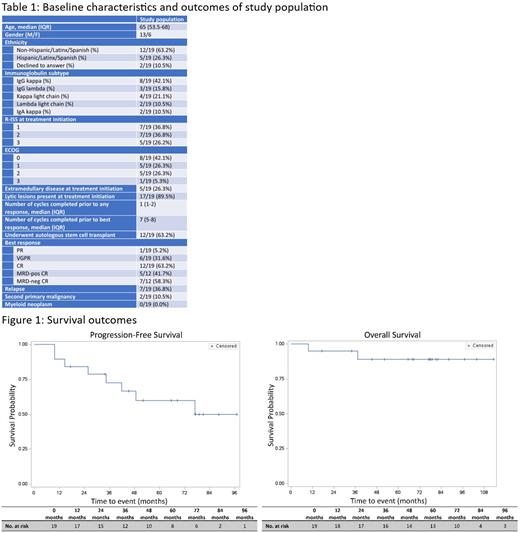
Data from 2/2014 to 7/2023 were reviewed. 19 patients received ≥2 cycles of KBD and were eligible for analysis. 5/19 (26.3%) had ≥1 high- or ultra-high-risk cytogenetic lesion (gain 1q, amplification 1q, 17p deletion/TP53 mutation, t(4;14), t(14;16), t(14;20)). 4 7/19 (36.8%) were R-ISS stage 1. 5/19 (26.2%) were R-ISS stage 3.
ORR was 100.0% with 12/19 patients (63.2%) achieving a complete remission (CR) and 7/12 (58.3%) minimal residual disease (MRD) negativity. Median time to response was 1 cycle and to best response 7 cycles. 12/19 patients (63.2%) underwent ASCT. Times to neutrophil and platelet engraftment occurred in the expected timeframe. 16/19 patients completed median 19 maintenance cycles (IQR: 1-24). 10/19 patients (52.6%) received carfilzomib 36 mg/m 2 every 2 weeks; 5 patients received lenalidomide d1-21 every 28 days; 1 patient received KRd. 2 patients declined maintenance; 1 patient died prior to maintenance due to septic shock.
After median follow up of 77.5 months (95% CI: 52.2-82.2), 7/19 (36.8%) patients suffered PD, including 3/12 (25.0%) who achieved CR. 1/19 died due to PD; 1/19 died due to septic shock. Median PFS was 77 months (34.5-NR); median time to next treatment (TTNT) was 77.0 months (36.1-NR). Median OS was not reached. 4/5 patients with high-risk cytogenetics suffered PD at median 48.6 months (14.6-NR); 1 without PD survived 17.2 months before loss to follow up. 1/5 high-risk cytogenetics patients died from PD at 37.2 months. For the 7/19 (36.8%) without ASCT, median PFS, TTNT, and OS were 41.8 months (9.8-NR), 45.2 months (9.8-NR), and not reached, respectively. 2/19 (10.5%) patients developed a second primary malignancy: melanoma in situ, fully resected; and localized prostate cancer at age 73, on active observation. No MDS occurred.
CONCLUSIONS and DISCUSSION
Patients with newly diagnosed MM who received KBD and, if eligible, ASCT had an excellent outcome with a median PFS of 77 months and a median OS that has not yet been reached at a median follow up of over 6 years. These findings equal or surpass the results achieved with RVD induction with a median PFS of 50 months for RVd + ASCT and 36 months for RVd only. 5 No adverse effects due to bendamustine were apparent, including development of MDS. KBD with anti-CD38 mAb should be explored as a viable IMID-sparing first-line regimen, regardless of ASCT eligibility.
REFERENCES
1. Mateos MV, Oriol A, Rosinol L, et al: Bendamustine, bortezomib and prednisone for the treatment of patients with newly diagnosed multiple myeloma: results of a prospective phase 2 Spanish/PETHEMA trial. Haematologica 100:1096-102, 2015
2. Jasielec JK, Kubicki T, Raje N, et al: Carfilzomib, lenalidomide, and dexamethasone plus transplant in newly diagnosed multiple myeloma. Blood 136:2513-2523, 2020
3. Leng S, Bhutani D, Raza S, et al: Phase I/II study of carfilzomib, bendamustine, and dexamethasone (CBD) in patients with newly diagnosed multiple myeloma. Blood Cancer J 10:13, 2020
4. Perrot A, Lauwers-Cances V, Tournay E, et al: Development and Validation of a Cytogenetic Prognostic Index Predicting Survival in Multiple Myeloma. J Clin Oncol 37:1657-1665, 2019
5. Attal M, Lauwers-Cances V, Hulin C, et al: Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N Engl J Med 376:1311-1320, 2017
OffLabel Disclosure:
Leng:Merck & Co., Inc.: Current Employment, Current equity holder in publicly-traded company. Chakraborty:Sanofi Pasteur: Consultancy; Adaptive Biotechnologies: Consultancy; Janssen: Consultancy. Bhutani:Sanofi: Consultancy, Research Funding. Mapara:Crispr/vertex: Consultancy; Incyte: Consultancy; Bluebird bio: Consultancy. Raza:Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kite: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Lentzsch:Janssen: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees; Celgene: Research Funding; Adaptive Biotechnologies: Consultancy, Membership on an entity's Board of Directors or advisory committees; Alexion Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bristol Meyers Squibb: Membership on an entity's Board of Directors or advisory committees; Regeneron: Honoraria; Pfizer: Consultancy; Oncopeptide: Membership on an entity's Board of Directors or advisory committees; Karyopharm Therapeutics: Membership on an entity's Board of Directors or advisory committees; Clinical Care Options: Honoraria; Caelum Biosciences: Membership on an entity's Board of Directors or advisory committees, Patents & Royalties: January 1, 2041; Sanofi: Research Funding.
Carfilzomib: use in newly-diagnosed multiple myeloma Bendamustine: use in newly diagnosed multiple myeloma

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal